The time frame for each step of the CASGEVY treatment journey is approximate and will vary per patient. The entire CASGEVY treatment journey could take up to a year. CASGEVY treatment is overseen by care teams at Authorized Treatment Centers (ATCs). Every ATC completed the onboarding process and associated training for CASGEVY. The ATC will determine which steps of the patient's care are inpatient or outpatient. Sites of care listed below are based on Trial 1 (SCD) and Trial 2 (TDT).1-3
CASGEVY is indicated for patients aged 12 years and older with SCD with recurrent vaso-occlusive crises or TDT4
1
Patient Identification and Evaluation
Timing varies per patient. Patient evaluation occurs at an ATC1,2,4
Healthcare providers at an ATC will confirm which patients are appropriate for CASGEVY. The ATC will perform a benefits investigation to confirm the patient’s insurance coverage and obtain prior authorization.
If you do refer a patient for treatment at an ATC, be sure to follow up with them to help confirm they are able to locate and find a provider at an ATC for further assessment and initiation of treatment.
Explore our hypothetical Patient Profiles to help determine if your patient may be ready to start CASGEVY.
Order placement
Once a patient is determined to be eligible for CASGEVY, the ATC will place an order through the Vertex Connects® portal, a secure online order management system.2
2
A healthcare provider at the ATC will determine the need for exchanges or transfusions. Red blood cell (RBC) exchanges or simple transfusions are recommended prior to mobilization and apheresis.3,4
Screen patients for HIV-1, HIV-2, HBV, HCV, and any other infectious agents in accordance with local guidelines before collection of cells for manufacturing. CASGEVY should not be used in patients with active HIV-1, HIV-2, HBV, or HCV.4
Discontinue disease modifying therapies (eg, hydroxyurea, crizanlizumab, voxelotor) 8 weeks before the planned start of mobilization.4
For patients with SCD, prior to the apheresis procedure, it is recommended that patients be transfused with a goal to maintain a hemoglobin S (HbS) level of <30% of total hemoglobin (Hb) while keeping total Hb concentration ≤11 g/dL.4
For patients with TDT, prior to the apheresis procedure, it is recommended that patients be transfused with a goal to maintain total Hb ≥11 g/dL.4
In Trial 1 for SCD:
- Patients underwent RBC exchanges or simple transfusions for a minimum of 8 weeks before the start of mobilization to reduce the number of circulating sickled cells and continued until initiation of myeloablative conditioning3,5
- RBC exchanges or simple transfusions were performed within 3 days of the start of mobilization to create a more stable interface for cell collection and decrease the risk of clotting and platelet aggregation, which can interfere with cell collection3,5
Hb=hemoglobin; HbS=sickle hemoglobin; HBV=hepatitis B virus; HCV=hepatitis C virus; HIV=human immunodeficiency virus.
3
Patients are required to undergo mobilization followed by apheresis to isolate the CD34+ HSCs needed for CASGEVY manufacturing. If the minimum dose is not met after manufacturing, the patient will need to undergo additional cycles of mobilization and apheresis to obtain more cells.4
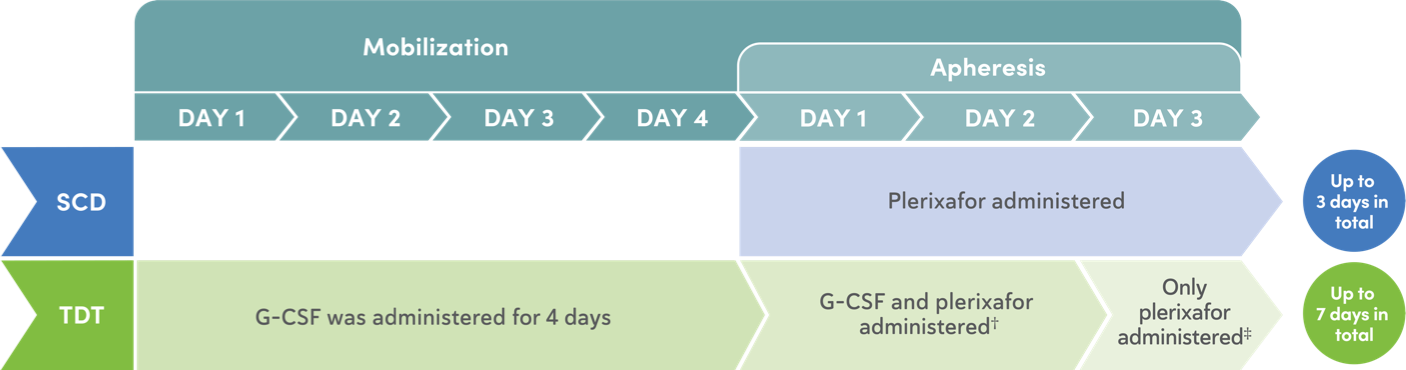
Mobilization
- Mobilization regimen increases yields of CD34+ HSCs prior to apheresis6
- For patients with SCD, only plerixafor was used for mobilization4
- For patients with TDT, G-CSF and plerixafor were used for mobilization4
Apheresis
Maximize CD34+ cell collection to obtain as many CD34+ cells as possible for CASGEVY manufacturing during each mobilization and apheresis cycle.4
Apheresis Days 1-2:
- Perform 2 consecutive days of cell collection for product manufacturing per cycle, if clinically tolerated. A total collection target of at least 20×106 CD34+ cells/kg is recommended for product manufacture3
- Collected cells to be used for the manufacture of CASGEVY are shipped each day from the ATC to the manufacturing facility. These cells cells should be sent for product manufacturing even if the total collection target is not achieved3,4
Apheresis Day 3:
- At least 2×106 CD34+ cells/kg is required to be collected for unmodified backup cells. A third day of cell collection can be used to obtain backup cells, if needed. If the target for backup cells is met, a repeat collection during any additional cycles will not be needed3,4
Backup cells
Unmodified backup CD34+ HSCs may be used for rescue treatment under any one of the following conditions4:
- Compromise of CASGEVY after initiation of myeloablative conditioning and before CASGEVY infusion4
- Neutrophil engraftment failure4
- Loss of engraftment after infusion with CASGEVY4
Additional mobilization and apheresis cycles
- If the minimum dose of CASGEVY (3×106 CD34+ cells/kg) is not met after initial product manufacturing, the patient will need to undergo additional cycles of mobilization and apheresis4
In Trial 1 and Trial 2:
- The median number of mobilization and apheresis cycles required was 2 (range: 1-6) for SCD in Trial 1 and 1 (range: 1-4) for TDT in Trial 2. In Trial 1, 6 (10%) patients were unable to receive CASGEVY therapy due to not achieving the minimum dose4
- All treated patients achieved neutrophil engraftment, and no patients received backup CD34+ HSCs4
Mobilization and apheresis timing in Trial 1 and Trial 2
HSC=hematopoietic stem cell.
4
During the CASGEVY manufacturing process, CRISPR/Cas9 technology is used to precisely edit HSCs at the erythroid-specific enhancer region of the BCL11A gene. Patients will not typically remain at the ATC during the manufacturing process. They usually return home until the next step of the process.4
Manufacturing process
- Once the HSCs are received at the facility, it takes approximately 5-6 months to manufacture and quality test CASGEVY before it is sent back to the ATC1,2,4
- Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown4
Quality release testing
After manufacturing CASGEVY, quality release testing is performed to confirm the product meets release criteria, including viability, purity, content, potency, sterility, and other safety release tests, before being shipped.7
5
Myeloablative Conditioning, Infusion, and Engraftment
SCD: approximately 6 weeks at an ATC1,4* | TDT: approximately 7 weeks at an ATC2,4*
Myeloablative conditioning should only start once the availability of the complete set of vials comprising the total dose of CASGEVY and unmodified backup cells has been confirmed. Infusion of CASGEVY occurs in 1 day, at a minimum of 48 hours and a maximum of 7 days after the last dose of myeloablative conditioning. After infusion of CASGEVY, monitor for neutrophil and platelet engraftment.1,2,4
Myeloablative conditioning: 4 days4*
- Myeloablative conditioning should only start once the availability of the complete set of vials comprising the total dose of CASGEVY and unmodified backup cells has been confirmed4
- Consider administration of anti-seizure prophylaxis with agents other than phenytoin prior to initiating busulfan conditioning. Consider prophylaxis for hepatic veno-occlusive disease (VOD)/hepatic sinusoidal obstruction syndrome prior to initiating busulfan conditioning4
- Stop iron chelation therapy at least 7 days prior to myeloablative conditioning4
In Trial 1 and Trial 2:
- Patients were admitted inpatient at the ATC and received 4 days of myeloablative conditioning with busulfan intravenously via a central venous catheter at a planned starting dose of 3.2 mg/kg/day once daily (qd) or 0.8 mg/kg every 6 hours (q6h)4
- Prophylaxis for hepatic VOD/hepatic sinusoidal obstruction syndrome was administered, per regional and institutional guidelines4
- All patients received anti-seizure prophylaxis with agents other than phenytoin prior to initiating busulfan conditioning4
Infusion
- Infusion of CASGEVY occurs in 1 day, at a minimum of 48 hours and a maximum of 7 days after the last dose of myeloablative conditioning1,2,4
- Administer CASGEVY through a central venous catheter via IV push4
- CASGEVY can cause serious hypersensitivity reactions due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion4
Engraftment and monitoring
- After infusion of CASGEVY, monitor for neutrophil and platelet engraftment.4
- In Trial 1 for SCD, the median (min, max) time to neutrophil engraftment in patients (N=44) was 27 (15, 40) days. The median time to platelet engraftment in patients (n=43) was 35 (23, 126) days4
- In Trial 2 for TDT, the median (min, max) time to neutrophil engraftment in patients (N=52) was 29 (12, 56) days. The median time to platelet engraftment in patients (N=52) was 44 (20, 200) days4
- There is potential risk of neutrophil engraftment failure after treatment with CASGEVY. In the clinical trials, all treated patients achieved neutrophil engraftment and no patients received rescue CD34+ cells4
- Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment4
- Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with backup CD34+ cells4
- Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise4
IV=intravenous.
6
Patients who received CASGEVY will have follow-ups post treatment, as determined by their healthcare providers.4
After CASGEVY administration
Standard procedures for patient management after HSCT should be followed4:
- Irradiate any blood products required within the first 3 months4
- Patients should not donate blood, organs, tissues, or cells at any time in the future4
- Restarting iron chelation may be necessary. Avoid the use of non-myelosuppressive iron chelators for at least 3 months and the use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used in lieu of iron chelation, when appropriate4
Understanding the steps of the CASGEVY treatment journey

Learn about the 6-step process with CASGEVY.
Voice-over:
Welcome to the CASGEVY®
INDICATION
CASGEVY is indicated for the treatment of patients aged 12 years and older with:
- sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs)
- transfusion-dependent β-thalassemia (TDT)
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
There is potential risk of neutrophil engraftment failure after treatment with CASGEVY. In the clinical trials, all treated patients achieved neutrophil engraftment and no patients received rescue CD34+ cells.
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
Please see full Important Safety Information at the end of this video and accompanying full Prescribing Information.
During this video, you will learn about the 6-step process for CASGEVY, a treatment for patients aged 12 years and older with sickle cell disease with recurrent vaso-occlusive crises or transfusion-dependent beta-thalassemia.
We will review the steps in the treatment process, including the important details for each step, how long they take, and where they occurred in the clinical trial. For the full breakdown, please refer to the Treatment Journey brochure, available for download by scanning the QR code on screen or at casgevyhcp.com
The time frame for each step of the CASGEVY treatment journey is approximate and will vary per patient with the possibility of the entire journey taking up to 1 year.
Step 1 is patient identification and evaluation.
Timing varies per patient and evaluation occurs outpatient at an Authorized Treatment Center, or ATC, which is a center that has completed the onboarding and training to provide CASGEVY.
Healthcare providers at an Authorized Treatment Center confirm which patients are appropriate for CASGEVY. Then a benefits investigation is performed to confirm the patient’s insurance coverage and obtain prior authorization. An order is then placed through the Vertex Connects®
Step 2 is pre-mobilization.
In the SCD clinical trial, RBC transfusions were required for a minimum of 8 weeks and occurred on an outpatient basis. In the TDT clinical trial, RBC transfusions were ongoing.
A healthcare provider at the Authorized Treatment Center will determine the need for simple transfusions or exchanges.
At this time, patients discontinue disease modifying therapies (such as hydroxyurea, crizanlizumab, and voxelotor) at least 8 weeks before the planned start of mobilization.
Step 3 is mobilization and apheresis.
For SCD, this step takes up to 3 days per cycle and was done inpatient in Trial 1. For TDT, this step takes up to 7 days per cycle and, in Trial 2, was done at a site location determined by the trial site on Days 1-4 and then was inpatient on Days 5-7.
Patients are required to undergo mobilization followed by apheresis to isolate the CD34+ hematopoietic stem cells needed for CASGEVY manufacturing.
Apheresis should be carried out for up to 3 consecutive days per cycle.
The overall goal is to collect as many cells as possible for the manufacture of CASGEVY and for the required collection of unmodified backup CD34+ hematopoietic stem cells.
If the minimum dose is not met after manufacturing, the patient will need to undergo additional cycles of mobilization and apheresis to obtain more cells. The median number of cycles required in Trial 1 for SCD was 2 (range: 1-6). The median number of cycles required in Trial 2 for TDT was 1 (range: 1-4).
Step 4 is manufacturing and quality.
It takes approximately 5-6 months to manufacture and quality test CASGEVY before it is sent back to the ATC. During this time, the patient is at home.
During manufacturing, CRISPR/Cas9 technology is used to precisely edit hematopoietic stem cells at the erythroid-specific enhancer region of the BCL11A gene.
Quality release testing is then performed to confirm the product meets release criteria, including viability, purity, content, potency, sterility, and other safety release tests, before being shipped back to the ATC.
Step 5 is myeloablative conditioning, infusion, and engraftment.
This step takes approximately 6 weeks for SCD and 7 weeks for TDT and occurred inpatient during the clinical trials.
Myeloablative conditioning should only start once the dose of CASGEVY has been received at the Authorized Treatment Center, and lasts for 4 days. It is important to discuss with your patients that myeloablative conditioning can cause infertility and be associated with other safety events.
Infusion of CASGEVY occurs in 1 day at a minimum of 48 hours and a maximum of 7 days after the last dose of myeloablative conditioning.
After infusion with CASGEVY, neutrophil and platelet engraftment should be monitored. Patients will remain in an ATC during this time. It is important to note here that there is potential risk of neutrophil engraftment failure after treatment with CASGEVY. Additionally, delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved.
Step 6 is follow-up.
After treatment, follow-ups are ongoing and will occur in an outpatient setting.
These follow-ups will be determined by the healthcare providers.
Vertex Connects offers support through Care Managers who will share educational resources to help patients prepare for each step, provide information and help answer questions along the treatment journey, and work with ATCs to help coordinate logistics of each patient’s treatment journey.
INDICATION
CASGEVY is indicated for the treatment of patients aged 12 years and older with:
- sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs)
- transfusion-dependent β-thalassemia (TDT)
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
There is potential risk of neutrophil engraftment failure after treatment with CASGEVY. In the clinical trials, all treated patients achieved neutrophil engraftment and no patients received rescue CD34+ cells.
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
ADVERSE REACTIONS
The most common Grade 3 or 4 non-laboratory adverse reactions (occurring in ≥ 25%) were mucositis and febrile neutropenia in patients with SCD and patients with TDT, and decreased appetite in patients with SCD.
All (100%) of the patients with TDT and SCD experienced Grade 3 or 4 neutropenia and thrombocytopenia. Other common Grade 3 or 4 laboratory abnormalities (≥ 50%) include leukopenia, anemia, and lymphopenia.
DRUG INTERACTIONS
No formal drug interaction studies have been performed. CASGEVY is not expected to interact with the hepatic cytochrome P450 family of enzymes or drug transporters.
Use of Granulocyte-Colony Stimulating Factor (G-CSF): G-CSF must not be used for CD34+ HSC mobilization of patients with SCD.
Use of Hydroxyurea: Discontinue the use of hydroxyurea at least 8 weeks prior to start of each mobilization cycle and conditioning. There is no experience of the use of hydroxyurea after CASGEVY infusion.
Use of Voxelotor and Crizanlizumab: Discontinue the use of voxelotor and crizanlizumab at least 8 weeks prior to start of mobilization and conditioning, as their interaction potential with mobilization and myeloablative conditioning agents is not known.
Use of Iron Chelators: Discontinue the use of iron chelators at least 7 days prior to initiation of myeloablative conditioning, due to potential interaction with the conditioning agent. Some iron chelators are myelosuppressive. If iron chelation is required, avoid the use of non-myelosuppressive iron chelators for at least 3 months and use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used instead of iron chelation, when appropriate.
USE IN SPECIFIC POPULATIONS
Pregnancy/Lactation: CASGEVY must not be administered during pregnancy and breastfeeding should be discontinued during conditioning because of the risks associated with myeloablative conditioning. Pregnancy and breastfeeding after CASGEVY infusion should be discussed with the treating physician.
Females and Males of Reproductive Potential: A negative serum pregnancy test must be confirmed prior to the start of each mobilization cycle and reconfirmed prior to myeloablative conditioning.
Women of childbearing potential and men capable of fathering a child should use effective methods of contraception from start of mobilization through at least 6 months after administration of CASGEVY. Advise patients of the risks associated with conditioning agents.
Infertility has been observed with myeloablative conditioning therefore, advise patients of fertility preservation options before treatment, if appropriate.
For more information about the CASGEVY treatment journey, scan the QR code.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
ADVERSE REACTIONS
The most common Grade 3 or 4 non-laboratory adverse reactions (occurring in ≥ 25%) were mucositis and febrile neutropenia in patients with SCD and patients with TDT, and decreased appetite in patients with SCD.
All (100%) of the patients with TDT and SCD experienced Grade 3 or 4 neutropenia and thrombocytopenia. Other common Grade 3 or 4 laboratory abnormalities (≥ 50%) include leukopenia, anemia, and lymphopenia.
DRUG INTERACTIONS
No formal drug interaction studies have been performed. CASGEVY is not expected to interact with the hepatic cytochrome P450 family of enzymes or drug transporters.
Use of Granulocyte-Colony Stimulating Factor (G-CSF): G-CSF must not be used for CD34+ HSC mobilization of patients with SCD.
Use of Hydroxyurea: Discontinue the use of hydroxyurea at least 8 weeks prior to start of each mobilization cycle and conditioning. There is no experience of the use of hydroxyurea after CASGEVY infusion.
Use of Voxelotor and Crizanlizumab: Discontinue the use of voxelotor and crizanlizumab at least 8 weeks prior to start of mobilization and conditioning, as their interaction potential with mobilization and myeloablative conditioning agents is not known.
Use of Iron Chelators: Discontinue the use of iron chelators at least 7 days prior to initiation of myeloablative conditioning, due to potential interaction with the conditioning agent. Some iron chelators are myelosuppressive. If iron chelation is required, avoid the use of non-myelosuppressive iron chelators for at least 3 months and use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used instead of iron chelation, when appropriate.
USE IN SPECIFIC POPULATIONS
Pregnancy/Lactation: CASGEVY must not be administered during pregnancy and breastfeeding should be discontinued during conditioning because of the risks associated with myeloablative conditioning. Pregnancy and breastfeeding after CASGEVY infusion should be discussed with the treating physician.
Females and Males of Reproductive Potential: A negative serum pregnancy test must be confirmed prior to the start of each mobilization cycle and reconfirmed prior to myeloablative conditioning.
Women of childbearing potential and men capable of fathering a child should use effective methods of contraception from start of mobilization through at least 6 months after administration of CASGEVY. Advise patients of the risks associated with conditioning agents.
Infertility has been observed with myeloablative conditioning therefore, advise patients of fertility preservation options before treatment, if appropriate.
Please see full Prescribing Information for CASGEVY.
References: 1. Data on file. Vertex Pharmaceuticals Incorporated. Boston, MA. REF-22852 (v3.0); 2023. 2. Data on file. Vertex Pharmaceuticals Incorporated. Boston, MA. REF-23044 (v3.0); 2024. 3. Data on file. Vertex Pharmaceuticals Incorporated. Boston, MA. REF-17378 (v1.0); 2022. 4. CASGEVY [prescribing information]. Vertex Pharmaceuticals Incorporated. Boston, MA; January 2024. 5. Protocol for: A phase 1/2/3 study to evaluate the safety and efficacy of a single dose of autologous CRISPR-Cas9 modified CD34+ human hematopoietic stem and progenitor cells (CTX001) in subjects with severe sickle cell disease. Vertex Pharmaceuticals Incorporated. Boston, MA. September 2021. 6. Yannaki E, Karponi G, Zervou F, et al. Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum Gene Ther. 2013;24(10):852-860. doi:10.1089/hum.2013.163 7. Data on file. Vertex Pharmaceuticals Incorporated. Boston, MA. REF-18762 (v10.0); 2023. 8. A long-term follow-up study in subjects who received CTX001. ClinicalTrials.gov identifier: NCT04208529. Updated January 2, 2024. Accessed January 12, 2024. https://clinicaltrials.gov/study/NCT04208529 9. Protocol for: A phase 1/2/3 study of the safety and efficacy of a single dose of autologous CRISPR-Cas9 modified CD34+ human hematopoietic stem and progenitor cells (hHSPCs) in subjects with transfusion-dependent β-thalassemia. Vertex Pharmaceuticals Incorporated. Boston, MA. July 2021.