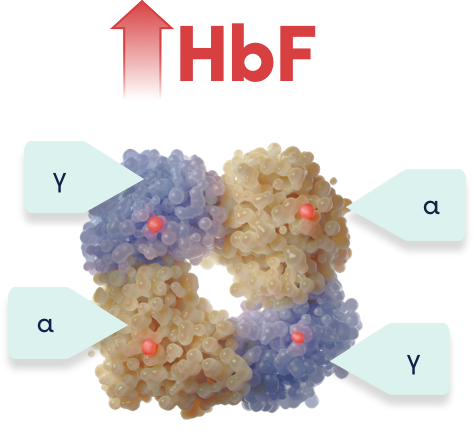
CASGEVY is made by targeting BCL11A to reactivate production of HbF, designed to mimic the HPFH phenotype1,2
CRISPR/Cas9 precisely targets the erythroid-specific enhancer region of the BCL11A gene using a nonviral delivery method1,3
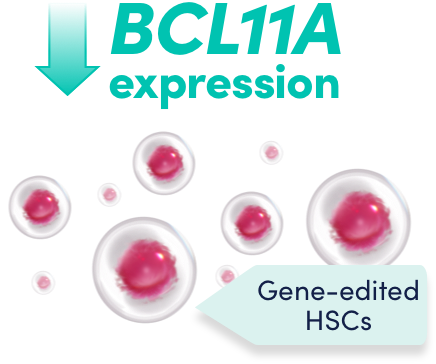
Gene-edited CD34+ HSCs have reduced expression of BCL11A specifically in erythroid lineage cells1
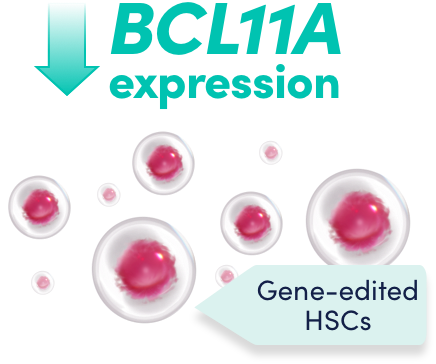
Reduced BCL11A expression leads to an increase in HbF levels in erythroid cells in vivo1
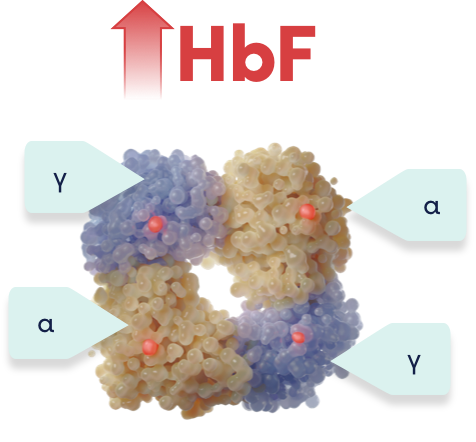
Increased HbF expression reduces intracellular HbS concentration, preventing the red blood cells from sickling1
CRISPR/Cas9 gene editing targets the erythroid-specific enhancer region of BCL11A to increase HbF1
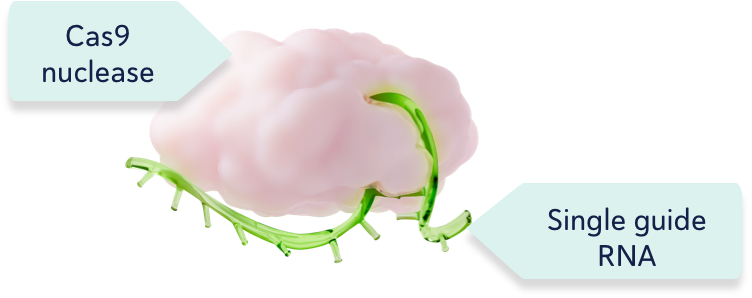
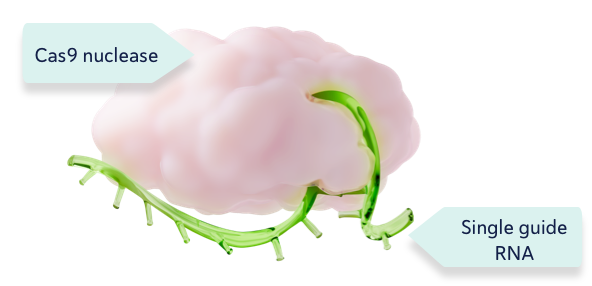
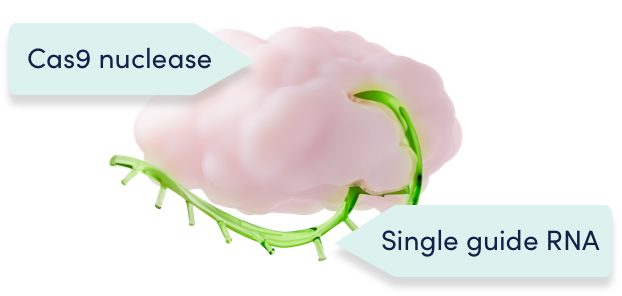
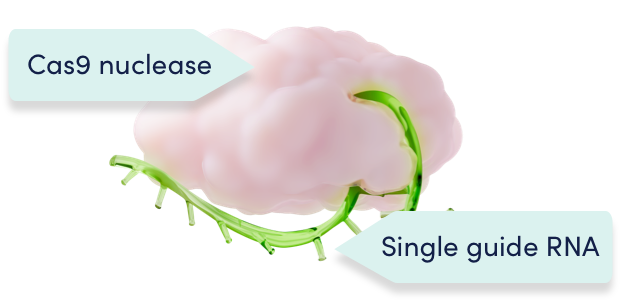
CRISPR/Cas9 consists of the Cas9 enzyme and a single guide RNA1,2
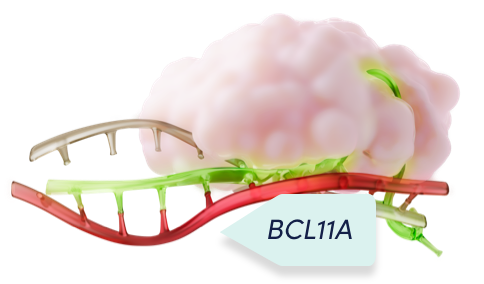
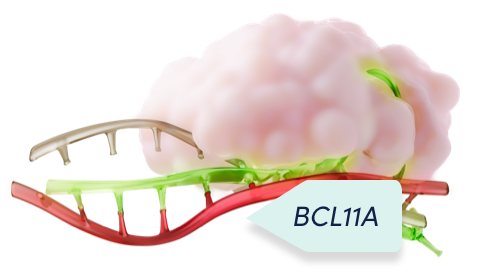
Cas9 and the single guide RNA function as a unit to precisely edit the target DNA1,2
During natural repair of the edited DNA, a change in the target DNA sequence is introduced1,2,4


The change to the target DNA sequence reduces BCL11A expression in erythroid lineage cells, leading to increased HbF production1
Features of CASGEVY
Hb=hemoglobin; HbF=fetal hemoglobin; HbS=sickle hemoglobin; HPFH=hereditary persistence of fetal hemoglobin; HSCs=hematopoietic stem cell; RNA=ribonucleic acid.
A closer look at how CASGEVY works

Watch how CASGEVY targets BCL11A to reactivate production of HbF, designed to mimic the HPFH phenotype.
Voice-over:
Discover CASGEVY (exagamglogene autotemcel): a first-of-its-kind, CRISPR/Cas9-modified cellular gene therapy.
INDICATION
CASGEVY is indicated for the treatment of patients aged 12 years and older with:
- sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs)
- transfusion-dependent β-thalassemia (TDT)
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
There is potential risk of neutrophil engraftment failure after treatment with CASGEVY. In the clinical trials, all treated patients achieved neutrophil engraftment and no patients received rescue CD34+ cells.
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Please see the Important Safety Information at the end of this video and full Prescribing Information at CASGEVYhcp.com.
Soon after birth, the production of fetal hemoglobin shifts to adult hemoglobin. This shift is mediated by increased BCL11A expression.
Patients with SCD or TDT have a mutation in the β-globin gene and the onset of disease symptoms typically coincides with the shift from fetal hemoglobin to adult hemoglobin.
In SCD, under the conditions of deoxygenation this mutation results in sickle hemoglobin polymerization, deforming red blood cells into a sickled shape, impairing blood flow and leading to vaso-occlusive crises and associated complications.
In TDT, the mutation leads to an absence or reduction of β-globin and a subsequent increase in unbound α-globin—ultimately reducing the number and function of red bloods cells, leading to anemia and the need for regular transfusions.
Patients with coinheritance of SCD or β-thalassemia and a condition called hereditary persistence of fetal hemoglobin (HPFH) continue to express high concentrations of fetal hemoglobin into adulthood and experience a milder clinical course.
CASGEVY was designed to mimic the phenotype of hereditary persistence of fetal hemoglobin, increasing levels of fetal hemoglobin with the potential to address the underlying cause of disease.
CASGEVY is manufactured ex vivo using a patient’s own CD34+ hematopoietic stem cells, or HSCs. The CD34+ HSCs are edited using a CRISPR/Cas9 complex introduced non-virally via electroporation.
A single-guide RNA directs the Cas9 enzyme precisely to the erythroid-specific enhancer region of the BCL11A gene and disrupts it, reducing BCL11A expression. Due to this precise approach, effects of BCL11A editing are only intended to occur in the red blood cells.
Once reintroduced into the patient’s body via a one-time infusion, the edited cells are expected to engraft in the bone marrow and differentiate to erythroid lineage cells with reduced BCL11A expression, which results in increased γ-globin expression and fetal hemoglobin production.
IMPORTANT SAFETY INFORMATION (continued)
WARNINGS AND PRECAUTIONS (continued)
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
In SCD, fetal hemoglobin expression can reduce intracellular sickle hemoglobin concentration, preventing the red blood cells from sickling, and thus avoiding occlusions.
In transfusion-dependent β-thalassemia, increased production of γ-globin may mitigate the α/non-α chain imbalance by pairing unpaired α-globin with γ-globin, producing fetal hemoglobin. This results in increased total hemoglobin levels, reduced ineffective erythropoiesis and hemolysis, and ultimately improved survival of red blood cells.
CASGEVY is a first-of-its-kind, CRISPR/Cas9-modified, autologous CD34+ cellular gene therapy. The CRISPR/Cas9 complex, delivered into the CD34+ HSCs ex vivo using a nonviral approach, edits the DNA at a precise location to increase fetal hemoglobin production. CASGEVY is a one-time infusion for patients who are 12 years of age or older with SCD with recurrent VOCs or TDT.
The treatment process includes mobilization and apheresis to collect CD34+ HSCs, editing CD34+ HSCs to manufacture CASGEVY, myeloablative conditioning, CASGEVY administration, and follow-up to monitor for engraftment and adverse events.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
There is potential risk of neutrophil engraftment failure after treatment with CASGEVY. In the clinical trials, all treated patients achieved neutrophil engraftment and no patients received rescue CD34+ cells.
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
ADVERSE REACTIONS
The most common Grade 3 or 4 non-laboratory adverse reactions (occurring in ≥ 25%) were mucositis and febrile neutropenia in patients with SCD and patients with TDT, and decreased appetite in patients with SCD.
All (100%) of the patients with TDT and SCD experienced Grade 3 or 4 neutropenia and thrombocytopenia. Other common Grade 3 or 4 laboratory abnormalities (≥ 50%) include leukopenia, anemia, and lymphopenia.
DRUG INTERACTIONS
No formal drug interaction studies have been performed. CASGEVY is not expected to interact with the hepatic cytochrome P450 family of enzymes or drug transporters.
Use of Granulocyte-Colony Stimulating Factor (G-CSF): G-CSF must not be used for CD34+ HSC mobilization of patients with SCD.
Use of Hydroxyurea: Discontinue the use of hydroxyurea at least 8 weeks prior to start of each mobilization cycle and conditioning. There is no experience of the use of hydroxyurea after CASGEVY infusion.
Use of Voxelotor and Crizanlizumab: Discontinue the use of voxelotor and crizanlizumab at least 8 weeks prior to start of mobilization and conditioning, as their interaction potential with mobilization and myeloablative conditioning agents is not known.
Use of Iron Chelators: Discontinue the use of iron chelators at least 7 days prior to initiation of myeloablative conditioning, due to potential interaction with the conditioning agent. Some iron chelators are myelosuppressive. If iron chelation is required, avoid the use of non-myelosuppressive iron chelators for at least 3 months and use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used instead of iron chelation, when appropriate.
USE IN SPECIFIC POPULATIONS
Pregnancy/Lactation: CASGEVY must not be administered during pregnancy and breastfeeding should be discontinued during conditioning because of the risks associated with myeloablative conditioning. Pregnancy and breastfeeding after CASGEVY infusion should be discussed with the treating physician.
Females and Males of Reproductive Potential: A negative serum pregnancy test must be confirmed prior to the start of each mobilization cycle and reconfirmed prior to myeloablative conditioning.
Women of childbearing potential and men capable of fathering a child should use effective methods of contraception from start of mobilization through at least 6 months after administration of CASGEVY. Advise patients of the risks associated with conditioning agents.
Infertility has been observed with myeloablative conditioning therefore, advise patients of fertility preservation options before treatment, if appropriate.
Please see full Prescribing Information for CASGEVY at CASGEVYhcp.com.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Neutrophil Engraftment Failure
Monitor absolute neutrophil counts (ANC) and manage infections according to standard guidelines and medical judgement. In the event of neutrophil engraftment failure, patients should be infused with rescue CD34+ cells.
Delayed Platelet Engraftment
Delayed platelet engraftment has been observed with CASGEVY treatment. There is an increased risk of bleeding until platelet engraftment is achieved. In the clinical trials, there was no association observed between incidence of bleeding events and time to platelet engraftment.
Monitor patients for bleeding according to standard guidelines and medical judgement. Conduct frequent platelet counts until platelet engraftment and platelet recovery are achieved. Perform blood cell count determination and other appropriate testing whenever clinical symptoms suggestive of bleeding arise.
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis can occur due to dimethyl sulfoxide (DMSO) or dextran 40 in the cryopreservative solution. Monitor patients for hypersensitivity reactions during and after infusion.
Off-Target Genome Editing Risk
Although off-target genome editing was not observed in the edited CD34+ cells evaluated from healthy donors and patients, the risk of unintended, off-target editing in an individual’s CD34+ cells cannot be ruled out due to genetic variants. The clinical significance of potential off-target editing is unknown.
ADVERSE REACTIONS
The most common Grade 3 or 4 non-laboratory adverse reactions (occurring in ≥ 25%) were mucositis and febrile neutropenia in patients with SCD and patients with TDT, and decreased appetite in patients with SCD.
All (100%) of the patients with TDT and SCD experienced Grade 3 or 4 neutropenia and thrombocytopenia. Other common Grade 3 or 4 laboratory abnormalities (≥ 50%) include leukopenia, anemia, and lymphopenia.
DRUG INTERACTIONS
No formal drug interaction studies have been performed. CASGEVY is not expected to interact with the hepatic cytochrome P450 family of enzymes or drug transporters.
Use of Granulocyte-Colony Stimulating Factor (G-CSF): G-CSF must not be used for CD34+ HSC mobilization of patients with SCD.
Use of Hydroxyurea: Discontinue the use of hydroxyurea at least 8 weeks prior to start of each mobilization cycle and conditioning. There is no experience of the use of hydroxyurea after CASGEVY infusion.
Use of Voxelotor and Crizanlizumab: Discontinue the use of voxelotor and crizanlizumab at least 8 weeks prior to start of mobilization and conditioning, as their interaction potential with mobilization and myeloablative conditioning agents is not known.
Use of Iron Chelators: Discontinue the use of iron chelators at least 7 days prior to initiation of myeloablative conditioning, due to potential interaction with the conditioning agent. Some iron chelators are myelosuppressive. If iron chelation is required, avoid the use of non-myelosuppressive iron chelators for at least 3 months and use of myelosuppressive iron chelators for at least 6 months after CASGEVY infusion. Phlebotomy can be used instead of iron chelation, when appropriate.
USE IN SPECIFIC POPULATIONS
Pregnancy/Lactation: CASGEVY must not be administered during pregnancy and breastfeeding should be discontinued during conditioning because of the risks associated with myeloablative conditioning. Pregnancy and breastfeeding after CASGEVY infusion should be discussed with the treating physician.
Females and Males of Reproductive Potential: A negative serum pregnancy test must be confirmed prior to the start of each mobilization cycle and reconfirmed prior to myeloablative conditioning.
Women of childbearing potential and men capable of fathering a child should use effective methods of contraception from start of mobilization through at least 6 months after administration of CASGEVY. Advise patients of the risks associated with conditioning agents.
Infertility has been observed with myeloablative conditioning therefore, advise patients of fertility preservation options before treatment, if appropriate.
Please see full Prescribing Information for CASGEVY.
References: 1. CASGEVY [prescribing information]. Vertex Pharmaceuticals Incorporated. Boston, MA; January 2024. 2. Frangoul H, Altshuler D, Cappellini MD, et al. CRISPR-Cas9 gene editing for sickle cell disease and β-thalassemia. N Engl J Med. 2021;384(3):252-260. doi:10.1056/NEJMoa2031054 3. Locatelli F, Lang P, Corbacioglu S, et al. Transfusion independence and elimination of vaso-occlusive crises after exagamglogene autotemcel in transfusion-dependent β-thalassemia and severe sickle cell disease. Presented at: 28th Congress of the European Hematology Association; June 8-15, 2023. 4. Doudna JA, Charpentier E. Genome editing: the new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346(6213):1258096. doi:10.1126/science.1258096